
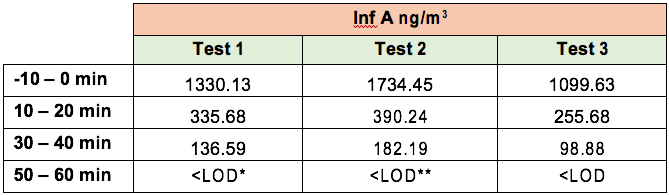
| Test Requested | Influenza A removal |
| Sample Description | Air Sniper Pro air cleaners |
| Number of Samples | 3 |
| Date of Receipt | 18 August 2016 |
| ASC Code | ASC003339 |
| Report Number | ASCR092180 |
| Report Date | 25 October 2016 |

Influenza virus infection is one of the most common and highly contagious infectious diseases and can occur in people of any age. Influenza A viruses are transmitted through direct contact, indirect contact, large respiratory droplets and aerosols (droplets nuclei).
Influenza viruses belong to the Orthomyxoviridae family and are divided into types A, B and C. Influenza types A and B are responsible for epidemics of respiratory illness that are often associated with increased rates of hospitalization and death. During the 20th century, the only influenza A subtypes that circulated extensively in humans were (H1N1) Spanish Flu; (H1N2); (H2N2) Asian Flu; and (H3N2) Hong Kong Flu. A new strain of influenza A, H1N1 emerged in 2009 called ‘Swine Flu’ as it originated in swine and spread to humans. More recently in 2013, a new strain of Avian Influenza A, H7N9 has infected people in China and is believed to be from exposure to infected poultry.
All known subtypes of influenza type A viruses have been isolated from birds and can affect a range of mammalian species. As with humans, the number of influenza A subtypes that have been isolated from other mammalian species is limited. Influenza type B viruses almost exclusively infect humans.
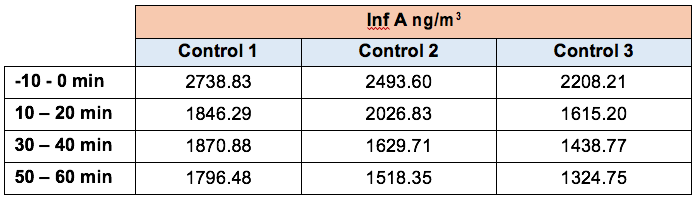
In this study influenza type A virus was used for the testing.
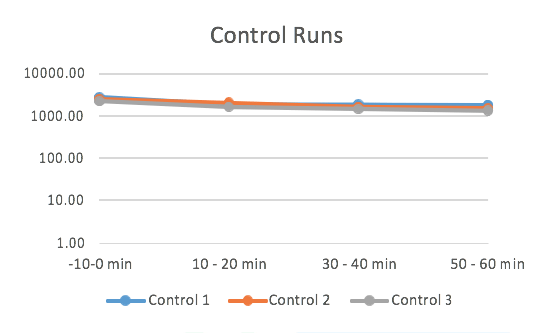
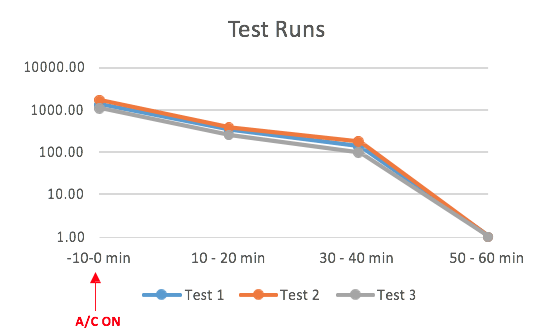
The concentration of Influenza A (Inf A) collected by the SKC BioSamplers was determined by ELISA (enzyme-linked immunosorbent assay).
ELISA samples were analysed according to the manufacturer’s specifications and samples were read at 450 nm on a BioTek EL 800 Plate Reader. The concentration of Inf A in each sample was quantified as ng per m3 of sampled air.
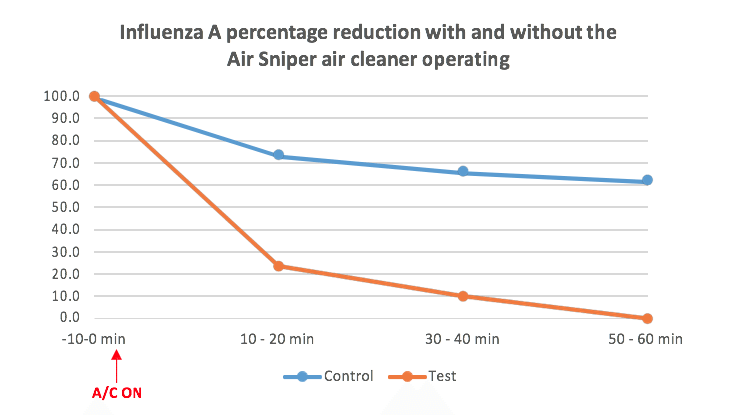
Virus reduction percentage was calculated according to the formula below:
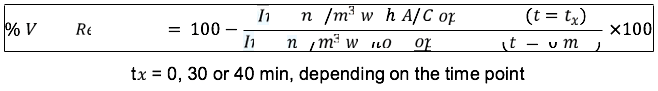
1) United States Environmental Protection Agency. EPA-454/B-13-001. Technical Assistance Document for the reporting of Daily Air Quality – The Air Quality Index (AQI). 2013.
2) World Health Organization. WHO Indoor Air Quality Guidelines: Formaldehyde, Chapter 5.8. WHO Regional Office for Europe, Copenhagen, Denmark. 2001
Call or email us, or fill out the form to have one of our customer service specialists get in touch with you.
603 S Governor Williams Hwy. Darlington, SC 29532
Phone: +1 (954)725-7500
Copyright 2022 Freshstart Medical Management.