Airmid Final Laboratory Report: Escherichia virus MS2 (Ultra Unit)

| Sample Description | Air Sniper Ultra Air Purifier |
| Number of Samples | 1 |
| Date of Receipt | 05 October 2020 |
| ASC Code | ASC004033 |
| Report Number | ASCR092438v2 |
| Report Date | 04 December 2020 |
1. Purpose
2. Test Item Description

3. Materials and Methods
3.1. 28.5 m3 Environmental Test Chamber
Testing was conducted in a state-of-the-art 28.5 m3 test chamber purpose-built to comply with the American Society for Testing and Materials (ASTM) standard. The chamber features include HEPA filtered supply air and an ability to maintain selected temperature and humidity levels at a wide range of air change rates. The chamber was constructed using powder-
coated stainless steel with all materials complying with low volatile organic compound (VOC) emission requirements.
The chamber is sealable from the exterior environment with access via an anteroom with interlocking doors and complies with cleanroom standards. The air change rate within the chamber can be controlled within a range of 0.5 to 20 air changes per hour.
3.2. Bacteriophage MS2 (MS2)
4. Protocol
4.1. Environmental Conditions
4.2. Air Purifier Active Test and Inactive Control Runs
Triplicate testing was conducted in the following configurations:
∙ Three inactive control runs without the air purifier operating
∙ Three active test runs with the Air Sniper Ultra 199-110200, placed on the floor in the centre of the test chamber, operating at speed setting 2.
In each active test run or inactive control run, viable MS2 virus was aerosolised into the test chamber for up to 30 minutes. Mixing fans were operated to promote homogenous distribution of the aerosol throughout the test chamber.
Testing was conducted in triplicate for both active test and inactive controls.
4.3. Air Sampling
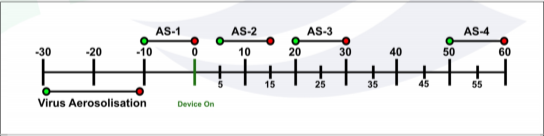
Air samples were collected at a 1.0-meter height from the floor at a rate of 11.5 l/m at the following timepoints:
∙ -10 to 0 minutes
∙ 5 to 15 minutes
∙ 20 to 30 minutes
∙ 50 to 60 minutes
4.4. Sample Analysis
5. Results
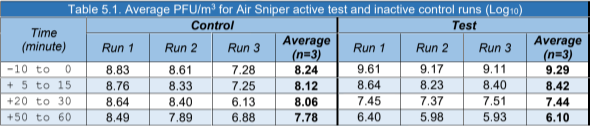
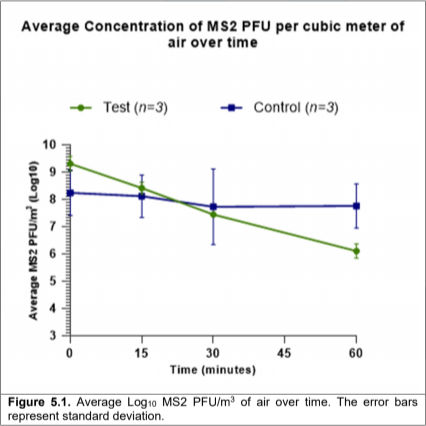
6. Discussion
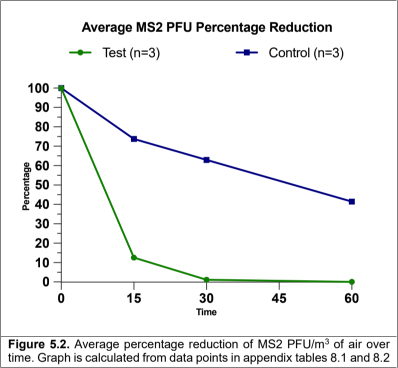
Our environmental test chamber assessment demonstrated that, when challenged with MS2, the Air Sniper Ultra 119-110200 air purifier was capable of reducing the average airborne concentration of the virus from 9.29 to 6.10 Log10 PFU/m3 after 60 minutes of operation.
The triplicate inactive control runs (no air purifier) did not show the same scale of reduction, the average concentration of MS2 decreased from 8.24 to 7.78 Log10 PFU/m3.
Calculating the percentage reduction based on the PFU/m3results there was a 99.9% reduction of airborne MS2 within 60 minutes of the air purifier operating.
7. References
1) John Zhang, Ph.D.; Doug Huntley; Andy Fox; Bryan Gerhardt; Al Vatine; John Cherne. Study of Viral Filtration Performance of Residential HVAC Filters. ASHRAE Journal, Vol. 62, no. 8, August 2020
2) Tung-Thompson G, Libera DA, Koch KL, de los Reyes FL III, Jaykus L-A (2015) Aerosolization of a Human Norovirus Surrogate, Bacteriophage MS2, during Simulated Vomiting. PLoS ONE 10(8): e0134277. https://doi.org/10.1371/journal.pone.0134277
3) G.W. Park, K.G. Linden, M.D. Sobsey (2010) Inactivation of murine norovirus, feline calicivirus and echovirus 12 as surrogates for human norovirus (NoV) and coliphage (F+) MS2 by ultraviolet light (254 nm) and the effect of cell association on UV inactivation. Letters in Applied Microbiology (Volume 52, Issue 2, Pages 162-167). https://doi.org/10.1111/j.1472- 765X.2010.02982.x
4) Jung Eun Lee, GwangPo Ko (2013) Norovirus and MS2 inactivation kinetics of UV-A and UV- B with and without TiO2. Water Research (Volume 47, Issue 15, Pages 5607-5613). https://doi.org/10.1016/j.watres.2013.06.035
5) EPA. 2006. “Generic Verification Protocol for Biological and Aerosol Testing of General Ventilation Air Cleaners.” Cooperative Agreement R-83191101. U.S. Environmental Protection Agency
6) Christopher M. Walker and GwangPyo Ko. Effect of ultraviolet germicidal irradiation on viral aerosols. Environ. Sci. Technol. 2007, 41, 5460–5465 https://doi.org/10.1021/es070056u
7) UV Lights and Lamps: Ultraviolet-C Radiation, Disinfection, and Coronavirus https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/uv-lights- and-lamps-ultraviolet-c-radiation-disinfection-and-coronavirus

